The COMBAT Group invests heavily in clinical trials to prove the Safety and Efficacy of our technologies.
We continue to work closely with clinicians on trials, evaluations and future developments to extend clinical use and optimise patient outcomes in an increasing number of disease areas.
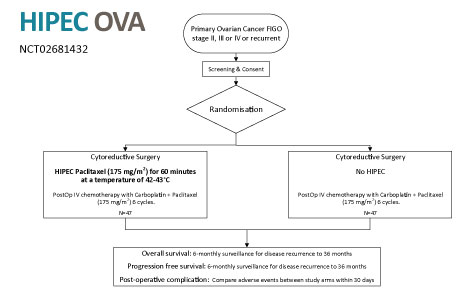
The PRS+ is currently being evaluated in the following randomised trials and clinically validated registries:Ovarian Cancer – NCT02681432
A randomised 72 patient, phase III clinical trial in women with epithelial primary ovarian cancer (Stage FIGO II, III and IV) or tumour recurrence.
https://clinicaltrials.gov/ct2/show/NCT02681432
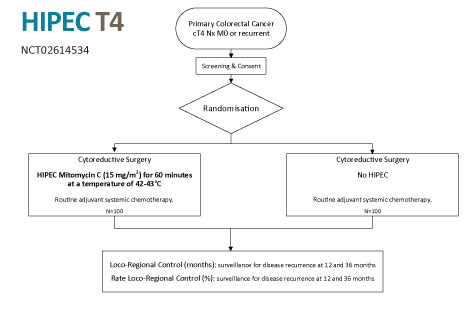
Colo-Rectal – HIPECT4 – NCT02614534
Multi centre, randomised, 200 patient phase II trial across 15 centres, evaluating the safety and efficacy of HIPEC with Mitomycin C (MMC) for treating locally advanced colorectal carcinoma. Expected completion 2021.
https://clinicaltrials.gov/ct2/show/NCT02614534
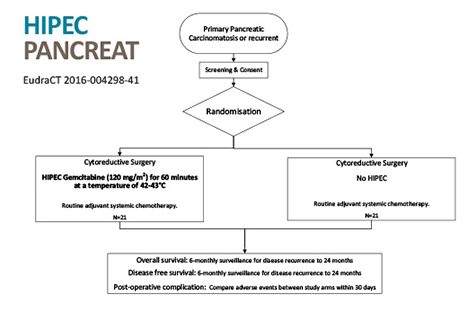
Pancreatic – Eurdract-2016-004298-41
A 42 patient, single centre, controlled, randomised, phase II trial using HIPEC with Gemcitabine in peritoneal carcinomatosis of pancreatic origin. Trial commenced 2017, expected completion 2021.
https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-004298-41/ES
Clinical Trial Flow Charts